Greifswald – Isle of Riems, 26.01.2017. - On 25 and 26 January the CCHFVaccine kick-off meeting took place at the Paul-Ehrlich-Institut. CCHFVaccine is a multinational and interdisciplinary research project. This consortium will investigate model vaccines against Crimean-Congo haemorrhagic fever for the protection of humans and animals. The Paul-Ehrlich-Institut and the Friedrich-Loeffler-Institut are among the 14 partners involved and will support the project with regulatory expertise as well as Biosafety Level 4 experimental capacity.
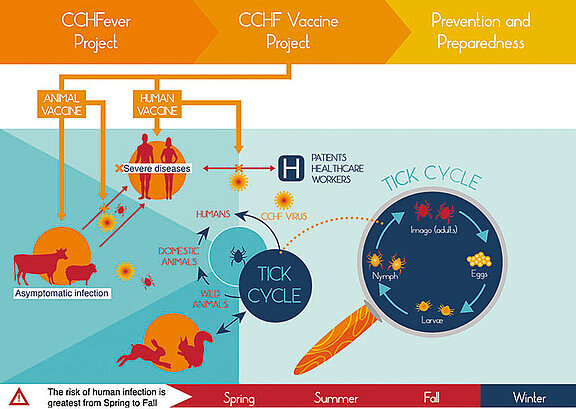
Crimean-Congo haemorrhagic fever is cause by the Crimean-Congo haemorrhagic virus (CCHFV). Ruminants constitute the primary viral reservoir. CCHFV is wide-spread and occurs in various countries in Europe, Asia, the Middle East, and Africa. The virus is transmitted by ticks and the close contact with infected blood or tissue. Infected animals generally do not show any signs of disease, whereas patients develop flu-like symptoms with fever, muscle and limb ache, as well as neurological and gastrointestinal signs. A subset of those affected develop serious haemorrhagies, and the mortality rate lies around 30 percent. Up to now, neither effective antiviral treatments nor licensed vaccines are available.
The CCHFVaccine Project aims at stepping up efforts to develop and provide vaccines against CCHFV for humans and animals thereby using an One Health approach as already exemplified by the participation of both major German medical and veterinary infectious disease research institutes. Vaccination of susceptible animals will be pursued to reduce the viral burden, whereas vaccine development for humans primarily aims at the protection of individuals at high risk for exposure. The project consortium is funded with six million € for a period of six years within the scope of the European research and innovation programme ‘Horizon 2020’ (see below).
The CCHFVaccine Consortium is led by Professor Ali Mirazimi at the Swedish Health Agency, the Karolinska Institute, and the Swedish National Veterinary Institute. The Paul-Ehrlich-Institut, which is the competent authority for the authorisation of clinical trials, marketing authorisations, and official batch control in Germany, will provide expert advice in regulatory aspects. The Friedrich-Loeffler-Institut, Federal Research Institute for Animal Health, Island of Riems will assess the efficacies of newly developed animal vaccines by challenging cattle in the unique newly commissioned BSL4 high containment laboratory and animal facility. The third German partner involved is the Justus-Liebig University, which is specialised in vaccine design.
Horizon 2020
Horizon 2020 is the framework programme of the European Union for research and innovation. It promotes research, and as such, aims at developing a knowledge- and innovation-driven society and a competitive economy. At the same time it has the purpose of contributing to sustained development. In addition to the member states of the European Union, various other countries can participate in the Horizon 2020 program.