Bovine Herpesvirus 1 (BHV-1) is also known as IBR or IPV virus and is the causative agent of the respective diseases (infectious bovine rhinotracheitis and infectious vulvovaginitis). Due to its economic significance, several European countries have made strong efforts to eliminate the disease from their cattle population. Some countries such as Denmark, Austria, South Tyrol or Switzerland already obtained the status „BHV-1 free“ pursuant to article 10 of EU directive 64/432/EEC years ago. In Germany, the control of BHV-1 infection has been regimented by law since Nov. 25, 1997 when the BHV-1 regulation came into force which made BHV-1 a notifiable disease in the Federal Republic of Germany. Main components are the vaccination with gE deletion vaccines and the targeted differentiation of vaccinated and field virus-infected cattle by detection of gE specific antibodies. Recently the designation of this type of vaccines has been changed to “DIVA vaccines” (Differentiating Vaccinated from Infected Animals) in the EU. Vaccination with these DIVA vaccines aims at eliminating field virus from affected regions and holdings without interfering with diagnostics.
However, this combination from DIVA vaccine and marker diagnostics creates new problems with respect to BHV-1 serology. To meet these challenges, the national reference laboratory for BHV-1 was founded at the Institute of Diagnostic Virology of the FLI on the island of Riems on January 3, 2000. This made Germany one of the first EU member states with a reference centre for this disease. A common EU reference centre does not exist so far. Several years ago, the NRL for BHV-1 was also designated as reference laboratory of the World Organisation for Animal Health (WOAH) so that it does not only serve as contact for federal and state authorities in Germany, but also provides assistance on the international level. In addition, the BHV-1 reference laboratory conducts research in the field of diagnostics, immunoprophylaxis and pathogenesis. The present developmental work concentrates on gE marker diagnostics and diagnostics for differentiation from closely related herpesviruses. But also marker-independent BHV-1 diagnostics from the neutralization test to the indirect BHV-1 antibody ELISA have been optimized and validated by the NRL over the past few years. The NRL for BHV-1 is accredited according to ISO17025.
The control of BHV-1 infection in Germany has made considerable progress. The first two regions (Upper Palatinate and Upper Franconia) have been declared BHV-1 free by the EU Commission pursuant to Article 10 of directive 64/432/EEC. Not only in Bavaria the percentage of so-called reagents has decreased to a very low level, also in other federal states with targeted vaccination programs (e.g. Saxony-Anhalt) there are hardly any gE positive holdings left. In 2008, 85.1 % of all dairy and suckler cow herds were considered BHV-1 or BHV-1-gE free.
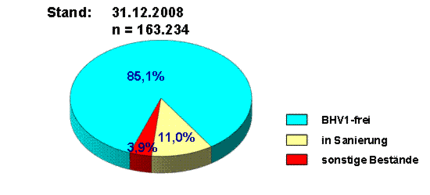
Thus, Germany is on a good way towards freedom from BHV-1 and we may expect that in the near future further regions will be declared BHV-1 free.
- Direct contact for German federal and state authorities for BHV-1 control related issues
- Antigen or genome detection in test materials
- BHV-1 antibody detection for verification of unclear results
- Supply of BHV-1 virus strains, reference sera and BHV-1 specific monoclonal antibodies
- Control, standardization and improvement of BHV-1 specific test methods
- Organization of qualification measures, e.g. for staff of veterinary diagnostic authorities
- Organization of national and international ring trials
- Participation in international and WOAH ring trials
- Participation in working groups and research projects of the European Union
- Research for improvement and standardization of BHV-1 diagnostics as well as on pathogenesis and immunization
- Virus detection in cell culture (virus isolation)
- Genome detection by real-time PCR and PCR
- Multiplex real-time PCR for differentiation of field and vaccine virus
- Strain characterization by restriction enzyme fragment length polymorphism (RFLP) analysis
- Differentiating neutralization and plaque reduction tests
- Antibody detection by neutralization test and ELISA: differentiating antibody analyses by gB blocking ELISAs, indirect ELISAs and gE-blocking-ELISAs
- Panel of more than 200 BHV-1 reference sera
- German BHV-1 reference sera R1 (positive), R2 (weak-positive), R3 (very weakly positive) as well as R31 and R32 (negative)
- German reference milk samples R26 (positive), R27 (weak-positive), R28 (positive), R29 (weak-positive), R39 (negative)
- Different BHV-1 reference strains
- collection of related herpesviruses (BuHV-1, BoHV-5, CvHV-1, CvHV-2, CpHV-1)

